Access is Restricted Your access to the NCBI website at www.ncbi.nlm.nih.gov has been temporarily disabled owing to an investigation into suspected misuse/abuse of your site. This is not a sign of a security vulnerability, such as a virus or an assault. It might be as easy as writing a runaway script or learning how to more efficiently utilize E-utilities, http://www.ncbi.nlm.nih.gov/books/NBK25497/, so that your work does not interfere with the capacity of other researchers to use our site. To regain access and learn how to engage more effectively with our site in the future, please contact info@ncbi.nlm.nih.gov through your system administrator.
Periodic Trends and the Periodic Table
Groups (vertical columns), periods (horizontal rows), and families comprise the periodic table (groups of elements that are similar). The valence electrons of elements belonging to the same group are identical. Meanwhile, atoms belonging to the same period have the same number of electron shells occupied. Dmitri Mendeleev, a Russian scientist, discovered in 1869 that chemical elements had an inherent pattern of organization. He derived the periodic table using this deduction. It is critical to observe how the placement of pieces in this table provides information about their attributes. Understanding the periodic patterns of an element is a rapid method to get an understanding of its chemical and physical characteristics. These patterns indicate the locations of the periodic table's greatest and lowest sorts of characteristics. To learn more about periodic patterns in more detail, go here.
Astatine
As with fluorine, astatine is the most uncommon of all the elements. Long after its existence was anticipated, scientists were unable to discover it in nature, and it was eventually discovered in 1940 when bismuth was bombarded with alpha particles (positively charged helium nuclei). The newly isolated element was given the Greek term âstability.â
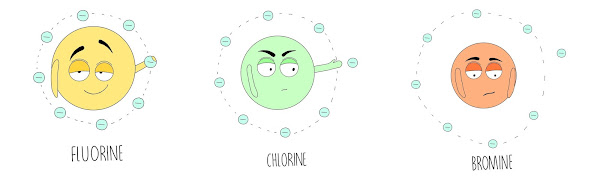
Henri Moissan, a French scientist, isolated fluorine (F, Z = 9) for the first time in 1886.
15 It is the most prevalent halogen on Earth's surface. 11 At room temperature, it is a light yellow gas that is exceedingly poisonous, as previously stated. Additionally, it is one of the most frequently utilized halogens. For instance, fluorine complexes are utilized in the creation of Teflon (a material that is used for anything from lining pots and pans to automobile components) and freons (which are used as coolants in refrigerators). Henri Moisson isolated fluorine for the first time in 1886.